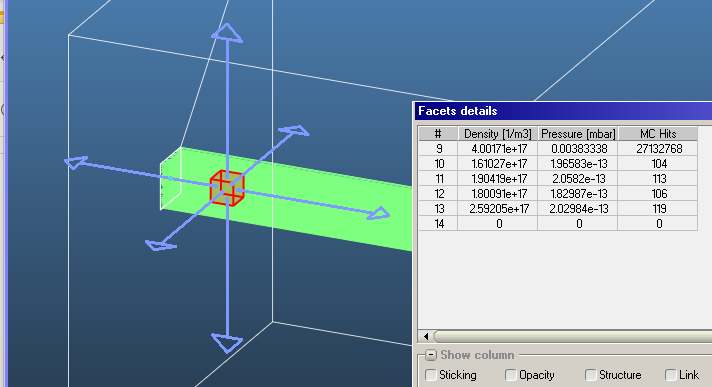
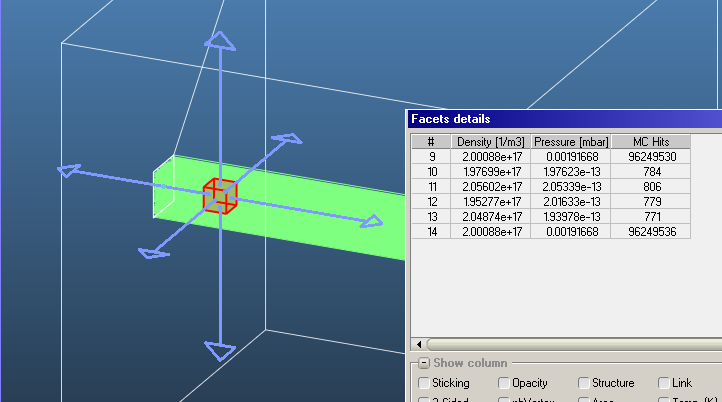
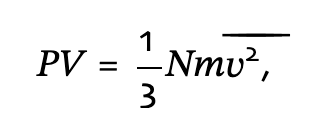Following up on the discussion of how you calculate density, I wanted to ask how you do this for pressure on transparent surfaces. Technically, pressures only exist on real surfaces as a force per unit area and can have components perpendicular and parallel to the surface. On the microscopic scale these forces are generated from the change in momentum imparted to the surface from particles that go to and from the surface. The outgoing particles and hence the pressure will depend on the surface properties: Temperature, Accommodation, Type of Reflection, Sticking Factor, Outgassing Rate. None of which applies to transparent surfaces. The common vacuum definition of pressure would require the gas to be an isotropic MWB. So from this stand point, if you were to place the equivalent real surface at the location of the transparent surface, then I assume you would have to take for the transparent surface…
Accommodation = 0
Sticking Factor = 0
Diffuse Reflection ?
to give the correct pressure for the common isotropic MWB case. Does this seem correct? Of course the gas doesn’t have to be isotropic or Maxwellian, which makes the interpretation of the pressure less straight forward. This is why I tend to think in terms of density and shape of the velocity distribution. Less likely to make mistakes when surface properties (Temperature, Sticking Factors, and etc…) are not so isotropic either globally or locally.
I assume you completely ignore particles coming from the backside, which may give you a different pressure. What do you do for both transparent and non-transparent two-side surfaces? Do you take an average?
Best,
Alan