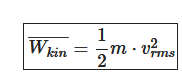Initial task,
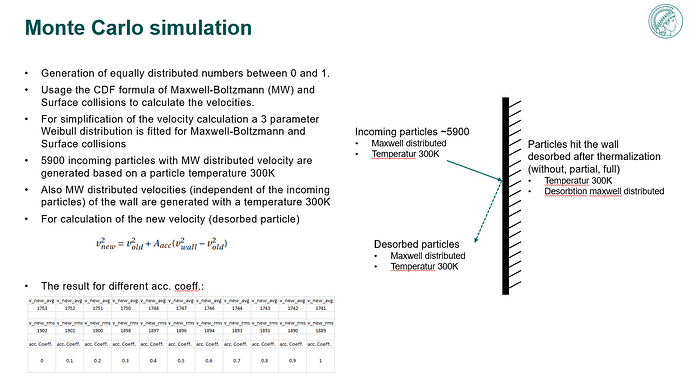
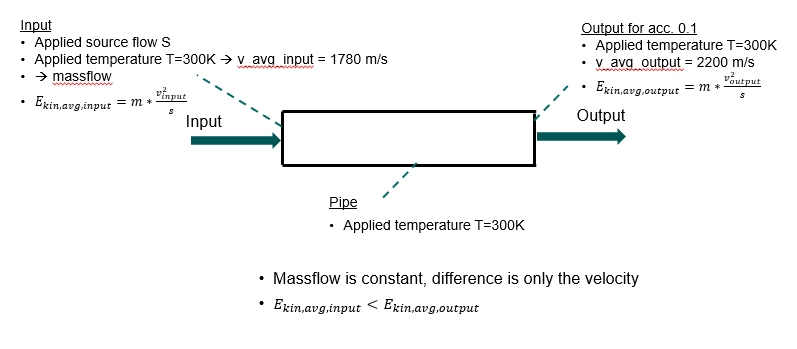
- analyse the impact of the accommodation by varying the temperature for a non-isothermal system
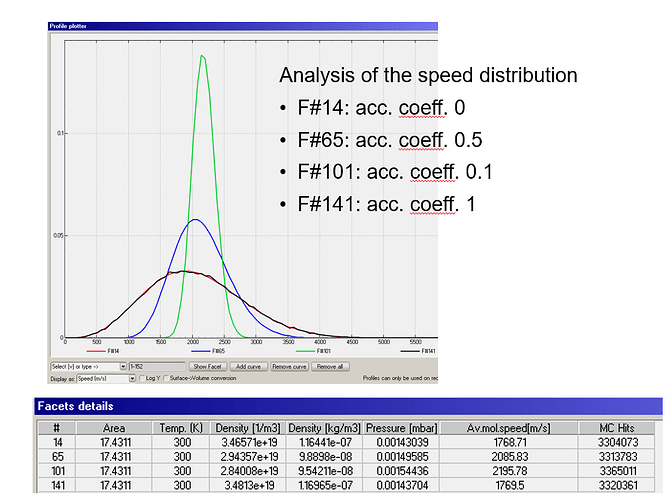
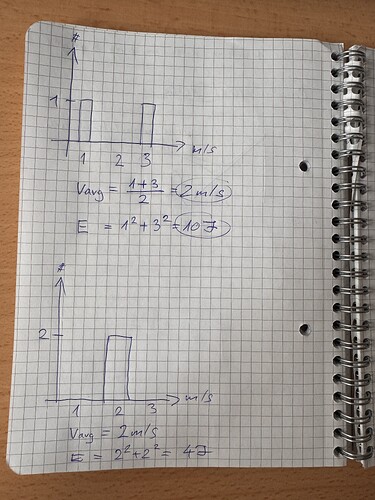
- useage of the average molecule velocity for analysis
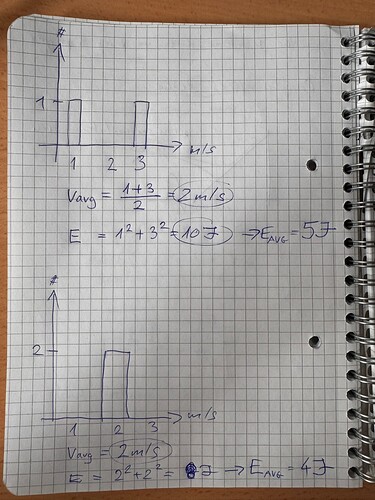
Simplified approach for plausibilisation
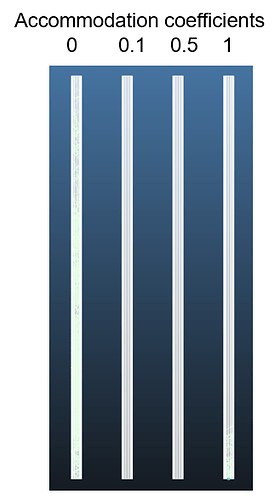
- isothermal system, all faces with the same temperature → expectation that the average molecule velocity inside the system must be the same
I already found this explanation, to be honest I didn’t really understand: Question on Accommodation Coefficient - #3 by maarton
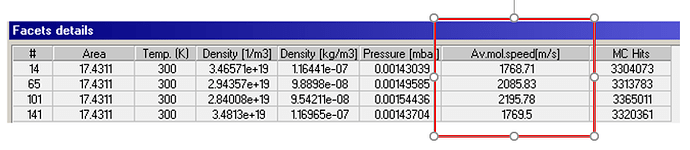
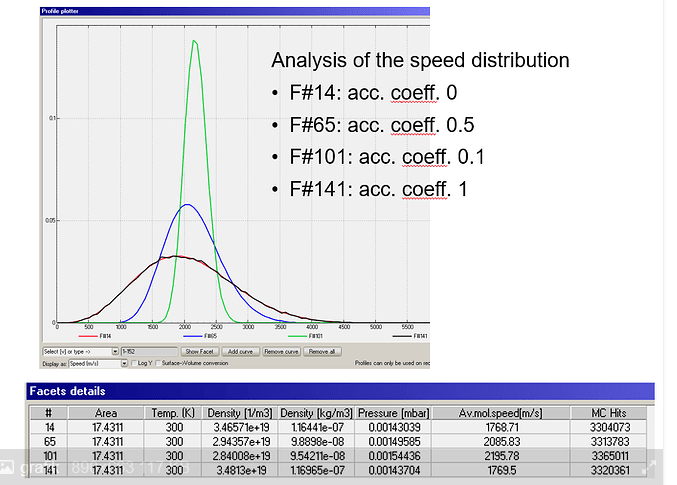
Isothermal system, example on 4 pipes
Questions:
-
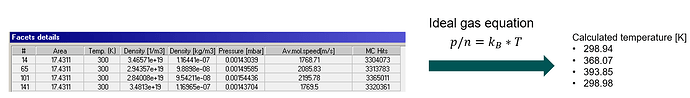
Main issue, as input I have 300K on every face, but my output depend on the accommodation coefficient getting different temperatures. I was expecting a temperature of 300K, because there is no additional energy input applied. How is this possible or why is it feasible?
-
Further more, what changes between accommodation coeff. 0 and 0.1?. It looks like there is a step between 0 and 0.1.
-
Calculating the temperature by the ideal gas equation or average mol. speed gives me higher temperatures for a coefficient 0 < coeff. < 1
Test_Velocity_AccommodationCoeff.zip (17.4 KB)
Version: molflow_win_2.9.25