Hello again,
It is not straightforward
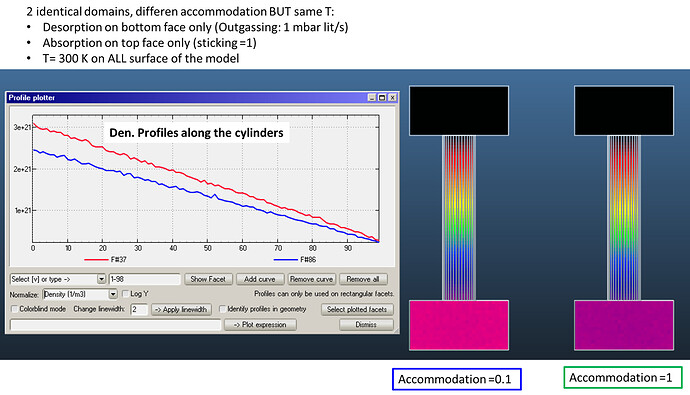
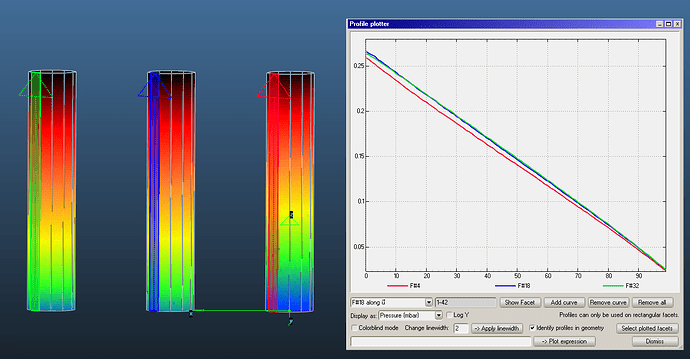
I have created three pipes with accomodation coefficients 1, 0.5 and 0.1.
You can see that the pressure is slightly larger for the low accomodation pipes:
This is due to a different velocity distribution:
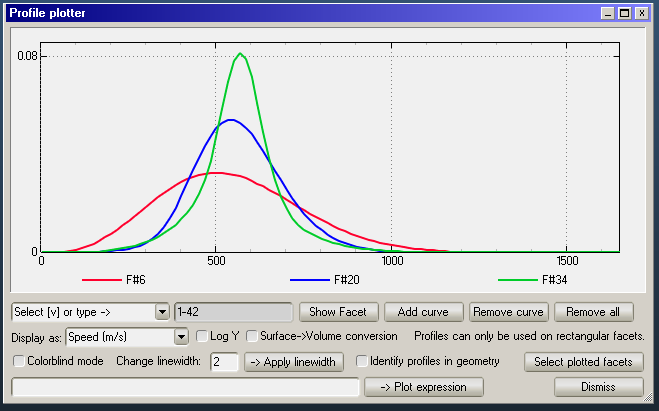
Low accomodation coefficient tubes have slightly faster molecules. The reason is that accomodation coefficient is defined in terms of energy, not speed:
New_energy = old_energy+(wall_energy-old_energy)*accomodation_coefficient
in simple terms, acc=1 will alwas assign “wall energy” (Maxwell-Boltzmann distribution according to wall temperature), acc=0 will keep old molecule speed, and acc=0.5 will take the average energy between old and wall.
Because of this, since E~v^2, the averaging tends to increase molecule speed. In plain simple English, fast moelcules lose speed more difficultly than slow molecules gain speed.
If you bear with me, a concrete numerical example for 4 molecules in a box, accomodation=50%.
In the beginning, the average speed is 2m/s, and for simplicity let’s assume E=v^2.
The molecules follow a dead simple velocity distribution: half of them are 1m/s and half 3m/s.
Initial speeds: 1m/s 1m/s 3m/s 3m/s (avg=2ms)
Inital energies: 1J 1J 9J 9J (avg=5J)
First collision:
old energies: 1J 1J 9J 9J (avg 5J)
new “wall” energies: 1J 9J 1J 9J (avg 5J)
thermalized energies after collision: 1J 5J 5J 9J (avg 5J)
thermalized speeds after collision ( sqrt(5)=2.236 ): 1m/s 2.236m/s 2.236m/s 3m/s (avg 2.118m/s)
As you can see even if the wall had the same distribution ans the initial, the collision shifted the velocity from 2m/s to 2.118m/s
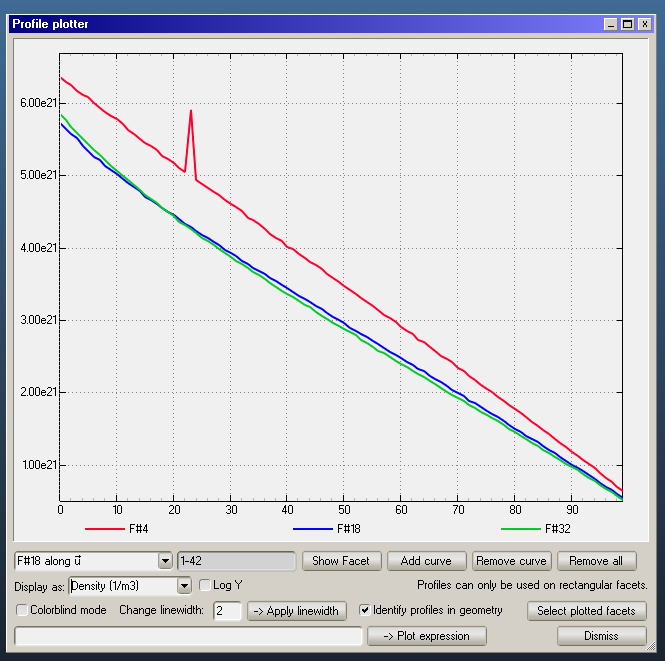
In case of density, the relation is the inverse of the pressure: slower molecules are more dense:
I will discuss with Roberto whether apart from the current accomodation definition (in terms of energy) we should introduce a new one (in terms of speed). This would require a bit of literature reading to see concrete examples of partial thermalization. If you let us know what you’re trying to simulate, maybe we can give advice for the best approach.
Hope I was clear, and thanks for bringing up the issue, Marton